Posted inNews
FDA Approves First and Only Twice-Yearly HIV Prevention Shot
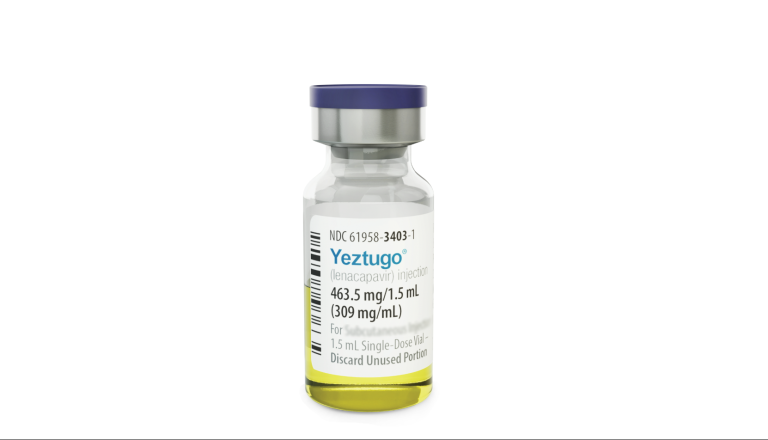
The U.S. Food and Drug Administration (FDA) has approved the first-ever twice-yearly injectable medication to prevent HIV. Branded Yeztugo® (lenacapavir) and developed by Gilead Sciences, this drug offers individuals a new option—just two subcutaneous injections per year—to reduce their risk of HIV infection [1][2].The injection contains the drug lenacapavir, a long-acting HIV medicine that’s been in development for years [5]. The FDA’s approval comes after results from major clinical trials showed the treatment was highly…